Effects of Eutrophication on Ranunculus and Potamogeton
Andrew J. Spink, K.J. Murphy, S.M. Smith and D.F. Westlake
Published in: Journal of Aquatic Plant Management 31, 113-117.
Abstract
Methods
Results & Discussion
Acknowledgements
References
Tables and Figure Captions
ABSTRACT
Water Crowfoot (Ranunculus penicillatus subsp. pseudofluitans
(Syme) S.Webster) plants were grown in two artificial recirculating rivers,
in one of which the phosphate concentration of the input was raised from 40 µgPl-1
to 200 µgPl-1. Fennel Pondweed (Potamgeton pectinatus L.)
plants were planted as a competitor in association with 50% of the Ranunculus
clumps. Chemical concentrations of the major elements in the water were measured
weekly. Filamentous algae grew in profusion in the channel with added phosphate
(0.77 T fresh weight), compared with an immeasurably low amount in the control
channel. After 100 days the plants were removed, dried and weighed and the tissue
concentrations of the major elements were measured. The Ranunculus shoots
grew less in the eutrophic channel, and its roots grew less in the presence of
Potamgeton. The Potamgeton showed a greater reduction in shoot and
root biomass than the Ranunculus. Tissue phosphate concentrations were
higher in both species in the eutrophic channel. The data suggested that P.
pectinatus is a more competitive species (sensu Grime) than R. penicillatus.
INTRODUCTION
River plants tend to be associated with particular nutrient concentrations
(Holmes and Newbold 1984). In streams where there is an inflow of polluted
water (for example, sewage effluent), species such as Ranunculus fluitans
L. and R. penicillatus are often replaced by Potamgeton pectinatus
(Butcher 1933, Harding 1979, 1980). Although the pollution is sometimes
intense enough to destroy the Ranunculus outright (Hawkes 1978), often
it appears that the change in vegetation is due to a change in the competitive
balance between the plant species present. However, there is a paucity
of experimental data to back up conclusions which have mostly been drawn from
field observations. The aim of the experiment described here was to investigate
the effects of pollution by increased phosphate concentration on R. penicillatus
subsp. pseudofluitans and Potamgeton pectinatus.
METHODS
The experiment was carried out in two artificial recirculating rivers at the
Waterston Experimental Station of the Institute of Freshwater Ecology.
Each artificial river consists of a 53 m long race-track shaped fibre(c)glass
channel, incorporating an Archimedes screw pump to circulate the water.
The water velocity was, on average, 0.25 m s-1 , with no significant
difference between the two channels. The channels were both filled
with water to a depth of 0.4 m above the gravel substratum. They have
a trapezoid cross-section, and the base was filled with gravel to a depth of
0.4 meters. The channels were continuously topped-up with groundwater
from a borehole. The input was adjusted to ensure that the volume of water
in each channel was 0.50 m when the gravel is installed (Fox, 1987). Bullhead
fish (Cottus gobio L.) were electro-fished from a nearby stream and placed
in the channels (equal numbers in each) to prevent large fluctuations
in populations of invertebrate grazers.
The water supply has a constant chemical composition which is similar to the
source of many chalk streams (Marker and Casey 1982, Casey and Newton 1973,
Westlake et al. 1972). It contained adequate concentrations of all the
ions necessary for plant growth, with the exception of iron (Marker and Casey
1982), so FeCl was added (together with EDTA) at a concentration equivalent
to 1 mg l-1 in the borehole water.
In one channel (the control) no other additional chemicals were added.
In the other, phosphate was added as phosphoric acid. The input PO-P concentration
was increased from 40 to 200 ug/l (see Figure 1). Concentrations of
200 - 750 ug/l have been measured in southern English chalk streams such as
the River Itchen which has abundant R. penicillatus
subsp. pseudofluitans (Spink 1992). The phosphate was continuously
added by a peristaltic pump from a 60 l vat. The major chemical elements
in the water were analysed weekly (Figure 1). Soluble phosphate and nitrate
was measured using flow injection analysis (Ruzicka and Hansen 1981), potassium
using atomic absorbtion spectrophotometry (American Public Health Association
1980) and sulphate was measured using an ion(c)exchange procedure (MacKereth
1955).
On 31 March 1990 R. penicillatus subsp. pseudofluitans
Potamgeton pectinatus plants were planted in the two channels.
Ten groups of five pots were planted with five Ranunculus plants in each
pot and an additional four
Potamgeton plants in 50% of the pots. The fresh weight of all the plants
was measured before planting out to ensure that
there was no initial difference between treatments. Before planting, water
was pumped for several hours between the two channels (in both directions, consecutively)
to ensure that the initial algal populations were similar for both channels.
On 12 July 1990 (after 100 days) the plants were removed from the channels,
dried (95 degreees C), separated into roots and shoots, weighed, and the tissue
concentrations of phosphorus, nitrogen, carbon and potassium were measured.
An estimate was also made of the weight of fillamentous algae (Cladophora
glomerata (L.) Kutz) in the channels.
A rigid polypropylene container (approximately 20 l) was carefully placed in
the channel and allowed to fill with water plus algae. The container was
removed from the channel, the volume of water was determined and the weight
of algae used to estimate the total weight in the total volume of the channel
(nine replicates).
RESULTS and DISCUSSION
The control channel had an immeasurably low amount of algae growing in it,
whereas the channel with added phosphate had an estimated 770 kg fresh weight
(23 kg dry weight) of fillamentous algae (Cladophora glomerata).
The channel with added phosphate had less Ranunculus shoot biomass, the
root to shoot ratio was increased and there was a greater concentration of major
nutrients in the shoots. There was no effect on the root biomass, but
this was decreased in the pots with Potamgeton pectinatus present.
The Potamgeton pectinatus root and shoot biomass was reduced in
the channel with added phosphate (though the ratio remained unaltered) and there
were reduced levels of other major nutrients (see Table 1
and Figure 2).
The addition of phosphate to one channel had a major effect on filamentous
algal growth in that channel. A likely result of this greatly enhanced
filamentous algal biomass would be to substantially reduce the quantity of light
available for submerged macrophyte photosynthesis, and so would be a significant
cause of stress (Turner et al. 1991, Marrs et al. 1992).
The ten pots in each channel were not true replicates but "pseudoreplicates"
as they were not statistically independent (Hurlbert 1984). As only two
channels were available for this experiment it was not possible to fully replicate
the treatment. The statistical comparisons are therefore comparisons between
the two channels rather than between
the two treatments. The question therefore arises as to whether it is
a reasonable assumption that the differences observed between the channels were
due to the phosphate treatment or due to another factor. The major measured
differences between the channels were the relatively high phosphate concentration
(Figure 1) and large algal
growth in the channel with the added phosphate; both were clearly directly caused
by the treatment. Conversely, the concentrations of many of the other
chemical elements rose and fell in concert in each channel during the
growing season (Figure 1; details in Spink 1992).
This indicates that it is likely that external factors acting on the channels
had
similar effects on each channel. However, although it is likely that the
effects on the plants observed were due to the treatment, the possibility that
it was due to another factor can not be excluded.
The data indicate that in the channel with the increased phosphate the Ranunculus
responded with a reduction in shoot growth (but no change in the root growth).
Conversely, the competition from the Potamgeton did not cause
any reduction in shoot biomass, but it did cause a significant reduction in
root growth. The reduced growth in both Ranunculus and Potamogeton
pectinatus was most likely to have been caused by shading which resulted
resulted from increased filamentous algal growth. This effect is
consistent with the hypothesis proposed by Phillips et al. (1978) to explain
the disappearance of aquatic macrophytes from the Norfolk Broads.
The tissue phosphate concentrations of both the Ranunculus and the Potamgeton
pectinatus were increased in the high phosphate channel. As the
sediment concentrations of nitrogen and potassium were identical in both channels
these data indicate that the concentration of these elements in the water is
a significant factor, demonstrating that, for both Potamgeton pectinatus
and R. penicillatus subsp. pseudofluitans , shoots as well as
roots are an important pathway for nutrient (N, P and K) uptake.
A number of characteristics of the biology of Potamgeton pectinatus
suggest that some ecotypes of this species show a strongly competitive strategy
(Grime 1979). P. pectinatus is frequently found in very productive
eutrophic habitats and can form an extensive dense canopy (Van Wijk 1988).
It shows little physiological acclimation to changes in light intensity, responding
instead with changes in biomass (Hootsmans and Vermaat 1991). The species
shows a strong seasonal variation in phenology and photosynthesis (Van Wijk
1988). From these characteristics it might be expected that Potamgeton
pectinatus would be a more competitive (and so less stress-tolerant)
plant than Ranunculus penicillatus - the data from this experiment
go some way towards confirming that. A competitive plant responds to stress
with relatively large changes in growth rate (Grime 1977, 1979), whereas a more
stress-tolerant plant will show a smaller change. The Potamgeton
shoot biomass was seven times smaller in the added phosphate ("stress") treatment,
whereas the Ranunculus shoot was only 4.4 times smaller (Figure 2).
In addition there was a significant biomass reduction in both the shoot and
root of the Potamgeton, whereas there was only a significant reduction
in the shoot of
Ranunculus.
In several English chalk streams dominated by R. penicillatus subsp.
pseudofluitans, the water phosphate concentrations have increased over
the past few decades. For example, the River Itchen at Winchester
has shown a three-fold increase in phosphate concentration during the period
1979 - 1989 (National Rivers Authority, unpublished data). The results
from this experiment indicate that, if the concentration of phosphate continues
to increase, it is likely that there may be a decline in macrophytes and an
increase in filamentous algae.
In the recirculating channels the algae could not get washed away downstream
and so this may have led to more algal accumulation than would occur naturally
in a fast-flowing river, though algal populations apparently as dense as those
in this experiment have been observed in some chalk rivers (Spink 1992).
In situations where algae may not grow so abundantly it would be useful to predict
what changes might occur in the balance between the species making up the macrophyte
community. Data from this experiment support the hypothesis that Potamogeton
pectinatus is a more competitive taxon than R. penicillatus subsp.
pseudofluitans (it showed larger mrophological changes
when stressed). So it is likely that if there was a situation of increased
nutrient supply without the stress caused by competition from filamentous algae,
the Potamgeton would forage nutrients more efficiently than the Ranunculus,
show a greater plasticity in its growth response, and so out-compete the Ranunculus.
Indeed, there is evidence (Caffrey
1990) to suggest that this has already happened in some organically polluted
rivers in the British Isles.
ACKNOWLEDGEMENTS
This experiment was carried out whilst AJS was holding a U.K. N.E.R.C. studentship.
The authors would like to thank Brian Dear for his help in maintenance of the
artificial rivers and Jean Lishman for assistance with plant chemical analysis.
LITERATURE CITED
American Public Health Association (1980). Standard Methods for the Examination
of water and wastewater, 15th Edition.
Butcher R.W. (1933) Studies on the ecology of rivers I. On the distribution
of macrophyte vegetation in the rivers of Britain. J. Ecol. 21: 58-91.
Caffrey J. (1990) Problems relating to the management of Potamgeton
pectinatus L. in Irish rivers. Proceedings EWRS 8th Symposium on
Aquatic Weeds, 1990, 61-68.
Casey H. and Newton P.V.R. (1973) The chemical composition of the River
Frome and its main tributaries. Freshwat. Biol. 3: 317-333.
Fox A.M. (1987) The efficacy and ecological impact of the management of
submerged macrophyte vegetation in flowing water. Ph.D. Theis, University
of Glasgow.
Grime J.P. (1977) Evidence for the existance of three primary strategies
in plants and its relevance to ecological and evolutionary theory. Am.
Nat. 111: 1169-1194.
Grime J.P. (1979) Plant Strategies and Vegetation Processes. John
Wiley and Sons Ltd, Chichester, UK. pp 222.
Harding J.P.C. (1979) River Macrophytes of the Mersey and Ribble basins,
Summer 1978. North West Water Authority Rivers Division, Scientists Department
Technical Support Group. Ref TS-BS-79-1.
Harding J.P.C. (1980) Macrophytes of the River Weaver. North West
Water Authority Rivers Division. Scientists Department Technical Support
Group. Ref TS-BS-80-2.
Hawkes H.A. (1978) River bed animals, tell-tales of pollution. In:
Hughes G. and Hawkes H.A. (eds) Biosurveillance of River Water Quality
pp 55-77. Proceedings of Section K of the BAAS, Aston 1977.
Holmes N.T.H. and Newbold C. (1984) River plant communities - reflectors
of water and substrate chemistry. Focus on Nature Conservation No. 9,
NCC, London.
Hootsmans M.J.M. and Vermaat J.E. (1991) Macrophytes, a Key to Understanding
Changes caused by Eutrophication in Shallow Freshwater Ecosystems. Report
Series 21. IIHEE, Delft, The Netherlands.
Hurlbert S.H. (1984) Pseudoreplication and the design of ecological field
experiments. Ecol. Mono. 54: 187-211
MacKereth F.J.H. (1955) Ion(c)exchange procedures for the estimation of
(i) total ionic concentrations (ii) chlorides and (iii) sulphates in natural
waters. Mitt. Int. Verein. Theor. Angew. Limnol. No. 4. 16pp
Marker A.F.H. and Casey H. (1982) The population and production dynamics
of benthic algae in an artificial recirculating hard-water stream. Phil.
Trans. Royal. Soc. B298: 265-308.
Marrs S.J., Murphy K.J. and Dominy P.J. (1992). Relationships between
submerged macrophytes and algae in freshwater lochs of differing trophic status.
Submitted to J. Aquat. Pl. Manage.
Phillips G.L., Eminson D. and Moss B. (1978) A mechanism to account for
macrophyte decline in progressively eutrophicated freshwaters. Aquat.
Bot. 4: 103-126.
Ruzicka J. & Hansen E.H. (1981) Flow Injection Analysis J. Wiley &
Sons, New York.
Spink A.J. (1992) The Ecological Strategies of Aquatic Ranunculus
Species. PhD Thesis, University of Glasgow. pp 340.
Turner M.A., Howell E.T., Summerby M., Hesslein R.H., Findlay D.L. and Jackson
M.B. (1991) Changes in epilithon and epiphyton associated with experimental
acidification of a lake to pH 5. Limnol. Oceanogr. 36(7): 1390-1405.
Van Wijk R.J. (1988) Ecological studies on Potamgeton pectinatus
L. I. General characteristics, biomass production and life cycles
under field conditions. Aquat. Bot. 31: 211-258
Westlake D.F., Casey H., Dawson F.H., Ladle M., Mann R.H. and Marker A.F.H.
(1972). The chalk stream ecosystem. Proc. IBP/UNESCO Symposium on Productivity
Problems of Freshwaters, Kazimierz Dolny 1970. Eds Kajak Z. and Hillbright-Ilkowska
A., pp 615-635.
TABLES
Table 1. Summary of Analysis of Variance.
"Competition" indicates the effects of Potamgeton pectinatus
plants on the Ranunculus plants, "Channel" indicates the difference between
the control channel and the one with added phosphate. Nutrient values
refer to levels in shoot tissue. If the value is indicated as an "increase"
it is greater in the treatment than in the control. Levels of significance
as follows: n.s. = not significant, * = 95%, ** = 99%, *** = 99.9%.
| Variate |
Source |
Significance |
Direction |
| Ranunculus |
|
|
|
| Shoot dry weight |
Competition |
ns |
|
|
Channel |
*** |
decrease |
|
Interaction |
ns |
|
| Root dry weight |
Competition |
* |
decrease |
|
Channel |
ns |
|
|
Interaction |
ns |
|
| Nitrogen |
Competition |
ns |
|
|
Channel |
*** |
Increase |
|
Interaction |
ns |
|
| Phosphate |
Competition |
ns |
|
|
Channel |
*** |
Increase |
|
Interaction |
ns |
|
| Potassium |
Competition |
ns |
|
|
Channel |
*** |
Increase |
|
Interaction |
ns |
|
| Potamogeton |
|
|
|
| Shoot dry weight |
Channel |
** |
Decrease |
| Root dry weight |
Channel |
* |
Decrease |
| Nitrogen |
Channel |
** |
Increase |
| Phosphate |
Channel |
*** |
Increase |
| Potassium |
Channel |
* |
Increase |
FIGURES
Figure 1
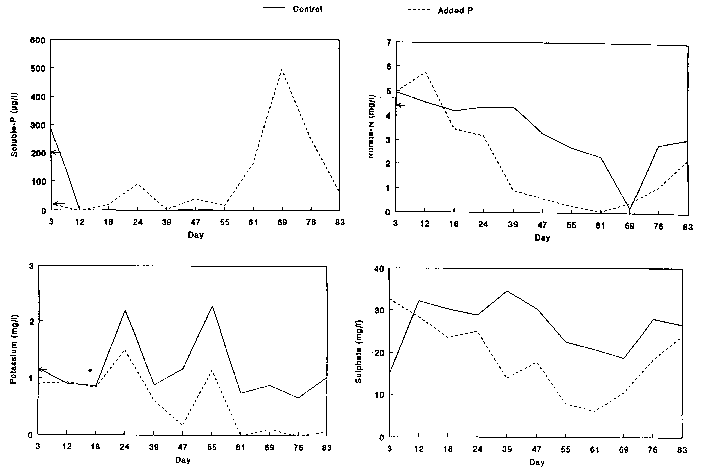
Chemical concentrations of soluble phosphate, nitrate, potassium and sulphate
in artificial rivers (clockwise from bottom left). Control channel shown
in solid line, channel with added phosphate
in dotted line. Concentration of input shown by arrow on ordinate (not
available for
sulphate).
Figure 2
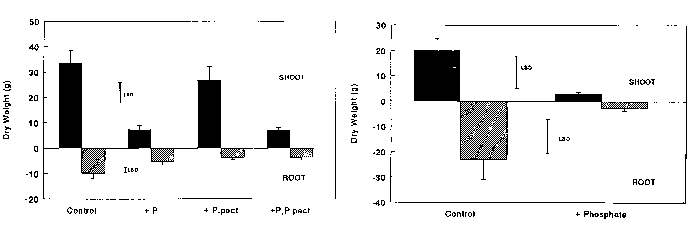
Effect of channel with added phosphate (+P) and presence of Potamgeton
pectinatus (+P.pect) on Ranunculus penicillatus subsp. pseudofluitans
growth (upper graph), and on P. pectinatus growth (lower graph).
"Control" represents plants in channel without added phosphate, and growing
without competition from P. pectinatus
. Bars on histograms represent ug/l 1 standard error, separate bars
represent least significant difference (LSD) (p ug/l
0.05).
Go to top


